SANTYL◊ Ointment versus silver-based products
62% mean reduction in ulcer area (n=51)**
SANTYL Ointment + supportive care* + periodic sharp debridement†
Overview
The primary objective was to compare the mean percent change in ulcer area from baseline to the end of the six-week treatment period in subjects receiving a daily application of SANTYL Ointment versus a comparator product containing silver.
- Phase 4, prospective, randomized parallel-group open-label study conducted at 14 sites in the United States and Canada
- Patients with Type 1 and 2 diabetes and nonischemic diabetic foot ulcers received once-daily SANTYL Ointment (n=51) plus supportive care* and periodic sharp debridement† or treatment with a silver-containing product‡ plus supportive care§ and periodic sharp debridement† (n=51)
- Subjects in both groups were instructed to replicate their assigned treatment daily throughout the six-week period
- All subjects in both groups were provided with an appropriate offloading device
Results
- The SANTYL Ointment group demonstrated a 62% mean reduction from baseline in ulcer area at week 6**, compared to 40% in the silver treatment group (P<0.071)17
- The secondary endpoints evaluated the number of subjects who experienced infection in the target ulcer area based on clinical signs and symptoms during the treatment period and evaluated the mean percent change in ulcer area from baseline to the end of the of the follow-up period (week 10).17
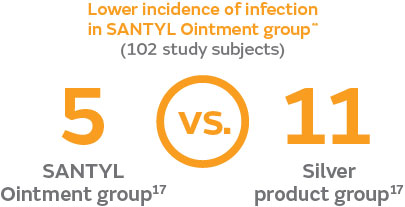
- Due to the enzymatic removal of necrotic tissue with SANTYL Ointment during the six-week treatment period, fewer ulcer infections were observed in SANTYL patients (n=5)** than in patients treated with silver-based products (n=11); (P=0.208)17¶
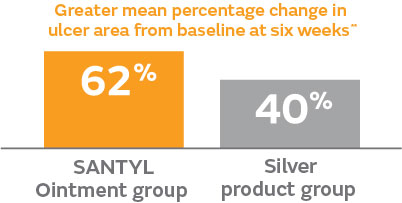
Four experts discuss clinically meaningful results revealed in the 2018 Motley study comparing clostridial collagenase ointment versus silver-containing product as adjuncts to sharp debridement in diabetic foot ulcers (DFU).
Conclusions
Mean percent reduction in DFU lesion area was greater with SANTYL Ointment plus supportive care* and periodic sharp debridement† compared to silver plus supportive care* and periodic sharp debridement†, as was time to ulcer closure, with an incidence of ulcer infection at least as low as the silver-containing product.
Read the full study in the October 2018 issue of Advances in Wound Care here.
*Supportive care in the SANTYL Ointment group included ALLEVYN◊ Foam Dressing, cast padding, and Coban™ or equivalent bandages.
†All subjects underwent sharp debridement prior to the application of the assigned test article and as medically necessary at any of the scheduled study visits.
‡Product containing silver was the investigator’s choice and was applied according to site procedures and the specific product’s Instructions for Use.
§Supportive care in the control (silver) group was the clinician’s choice. **Not statistically significant.
¶SANTYL Ointment is not indicated for the treatment or prevention of infection.

